
In the high-voltage power transmission world, safety and efficiency hinge on one critical component: the insulator. But what makes materials like glass perfect for this task?
The answer lies in the atomic structure and bonding properties of these materials. Let’s explore how glass insulators work and why they’re trusted to keep our global energy infrastructure running safely.
Understanding Electric Current and Material Behavior
Electric current is the flow of electric charge, usually carried by electrons, through a conductive material when it’s subjected to a voltage. In the power transmission world, controlling this flow is critical to avoid dangerous leaks and ensure grid efficiency.
All materials are made of atoms, composed of:
- A nucleus (protons and neutrons)
- Electrons orbiting the nucleus
The valence electrons, those in the outermost shell, determine how well a material conducts electricity. Their ability (or inability) to move between atoms sets conductors apart from insulators.
Copper vs. Glass: How Atomic Behavior Changes Everything
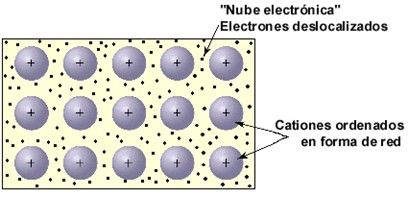
Conductive Materials (Copper)
- Valence electrons move freely between atoms
- This is made possible by metallic bonding, creating a “sea of electrons”
- As a result, electric current flows easily
Insulating Materials (Glass)
- Has valence electrons that are tightly bound
- Exhibits covalent bonding, where electrons are shared but not freed
- Lacks the free movement required for conduction, making it a natural insulator
Why Glass Is a Perfect Insulator
The insulating properties of glass come down to two main physical characteristics:
1. Atomic Structure
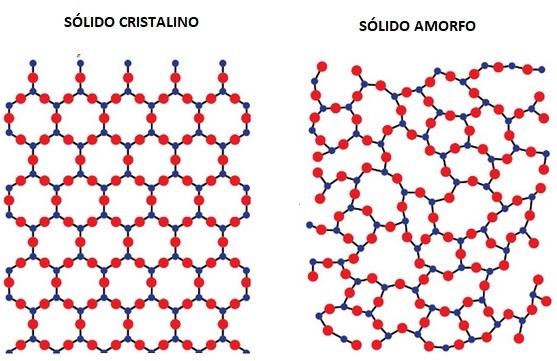
- Metals have a crystalline structure, where atoms are arranged in a regular, repeating pattern. This order makes it easier for electrons to flow.
- Glass has an amorphous structure, meaning its atoms are disordered and irregular. This disorder requires much more energy for electrons to move, effectively blocking current.
2. Type of Bonding
- In glass, the covalent bonds between atoms lock valence electrons into place. Even under high voltage, they don’t move, meaning no current can pass through.
- In copper, metallic bonds allow electrons to move freely, ideal for conduction, but not for insulation.
The Design of a Glass Insulator
A typical glass insulator is built for strength, durability, and maximum electrical resistance. It consists of:
- Two metallic parts: a pin/bolt and a cap
- One central glass unit, acting as the insulating barrier
- Aluminous cement joins the components, ensuring an electrically insulated seal
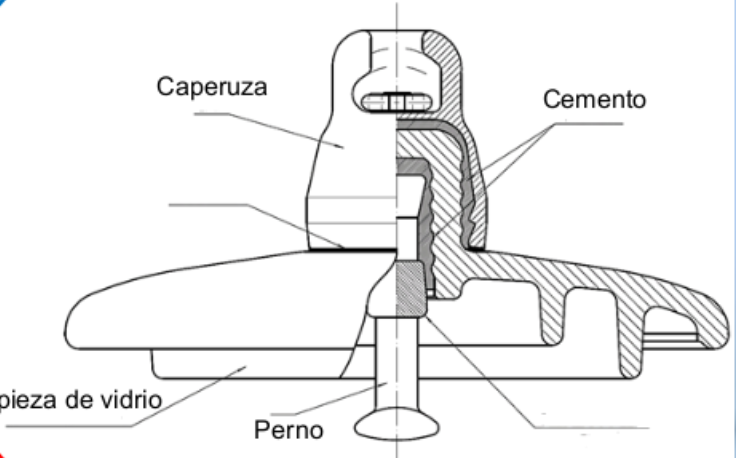
Engineering Insight: The glass head is the thickest and most critical part of the insulator. Each millimeter of glass can resist 20kV of electrical tension. Our standard La Granja models range from 10–15 mm, meaning each insulator can withstand 200kV or more.
The Role of Glass Insulators in Power Transmission
In practical terms, a glass insulator separates live cables from grounded metal structures (such as transmission towers). Without this separation:
- Electricity could flow into the tower and reach the ground
- This can cause short circuits, energy losses, or even electrocution
With a properly engineered glass insulator:
- Current remains confined to the transmission cable
- Power is safely transmitted over long distances, even in harsh environments
Key Takeaways: Why Glass Insulators Excel
- Covalent bonding: Keeps electrons locked in place, preventing electric current flow.
- Amorphous atomic structure: Irregular atomic arrangement blocks conduction.
- High mechanical strength: Withstands environmental stress like wind, ice, and heat.
- Visible damage detection: Cracks are instantly noticeable, simplifying inspection.
- Non-aging and UV resistance: Delivers long-term reliability with minimal maintenance.



